
Cayman’s Bioanalysis & Assay Development Services help validate an assay to effectively monitor SARS-CoV-2 neutralizing antibodies in people.
The ability to detect antibodies that neutralize SARS-CoV-2 and monitor seroprevalence is fundamental for the assessment of herd immunity, protective immunity durability, vaccine candidate efficacy, and therapeutic development. Many commercial ELISA-based assays produced in response to the COVID-19 pandemic to detect IgG or IgM antibodies can be inaccurate and do not evaluate virus neutralization that is correlative to protection.
Cayman scientists helped to validate an assay that allows direct assessment of the capacity of inhibitory immunoglobins or synthetic compounds to block the binding of spike protein to ACE2. A full report on the assay has been published in the Journal of Clinical Microbiology.
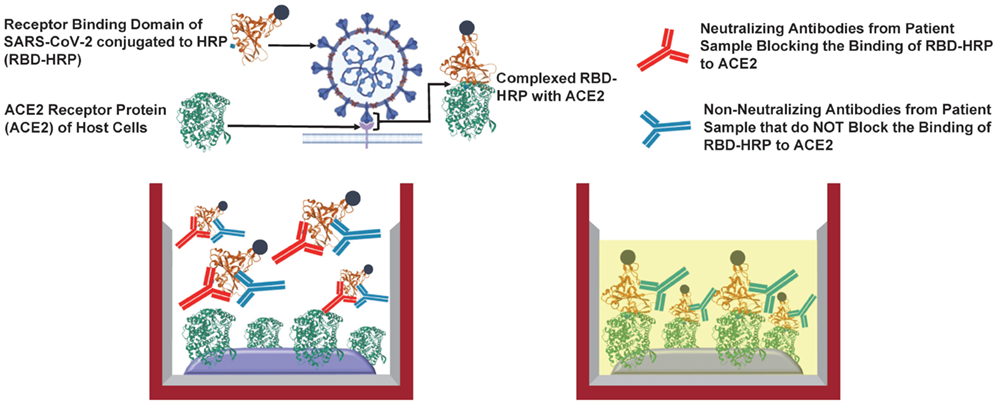
Schematic of assay design as described in Taylor, S.C., Hurst, B., Charlton, C.L, et al. A new SARS CoV-2 dual purpose serology test: Highly accurate infection tracing and neutralizing antibody response detection. J. Clin. Microbiol. 02438-20 (2021). (CC BY 4.0)
Cayman’s Contract Services division has been working on many fronts to support coronavirus research.
-
Assay Development – Detection of SARS-CoV-2 neutralizing antibodies.
-
Immunopeptidome Profiling – Identification of viral antigens presented by MHC complexes to T cells.
-
Lipidomics – Analysis of eicosanoids and oxylipins to detect putative biomarkers of inflammation in COVID-19.
-
SARS-CoV-2 Screening Library – Cayman can build a custom library with a diverse set of FDA-approved and drug-like compounds identified from in silico modeling to target ACE2, spike glycoprotein, main protease, and other crucial targets.